Inclusivity and clinical trial safety
Clinical trial participation that represents the diversity of people in our population leads to safer and more effective treatments.
How diverse participation leads to safer treatments
Clinical trials that include people from diverse backgrounds lead to treatments and vaccines that are safer and more effective. When we only include certain groups, we don’t learn enough about how safe treatments are for everyone. Imagine if only one type of person tested a potential medicine. We wouldn’t know if it works the same for others. When a trial includes diverse groups, we can have greater confidence a potential treatment is safe and effective across all people.
Representing all our populations in clinical research
People from different racial, ethnic, and demographic backgrounds might experience different outcomes in response to treatments and vaccines. Some people may experience changes in their health that others don’t. There may be side effects in some groups but not others. Diverse participation in clinical trials help us learn about health outcomes across all groups. A great deal of data shows that clinical trials need much more diversity among participants.
Race
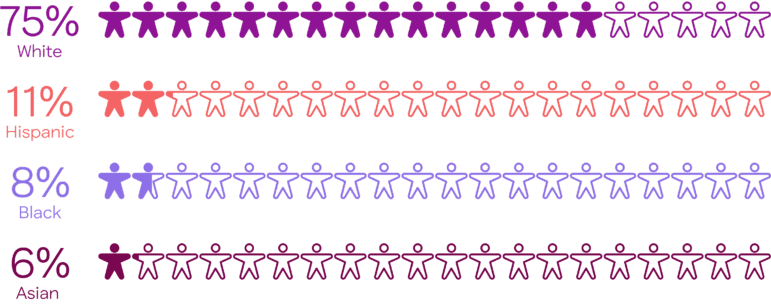
In 2020, 75% of clinical trial participants were white people, despite being only 60% of the U.S. population. People of color make up about 40% of the U.S. population. But they are only 25% of clinical trial participants.
These numbers show troubling gaps in clinical trial participation. We need to close these gaps. Trial sponsors must have plans for reaching diverse groups. Healthcare providers must talk about trials with patients from all backgrounds. And community leaders can help by spreading the word about trials.
Biological sex and gender
For decades, clinical trials were not required to include females. In 1993, a law was finally passed requiring that trials include them. More than 30 years later, we still have work to do. Females make up more than half the population. Yet, they make up only 41% of trial participants. On the other hand, males make up 59% of trial participants. The gap has narrowed, but more females are needed in trials.
Age
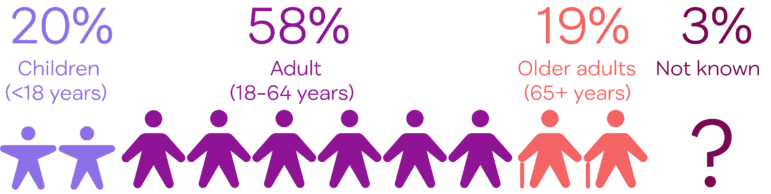
Adults aged 18-64 make up more than half of clinical trial participants. People in this age range are less likely to have health conditions that might keep them out of a clinical trial. It can also be easier for them to complete trial activities. Older adults and children tend to require more support to participate in a trial. Trial sponsors must address age-based challenges.
Geographic location
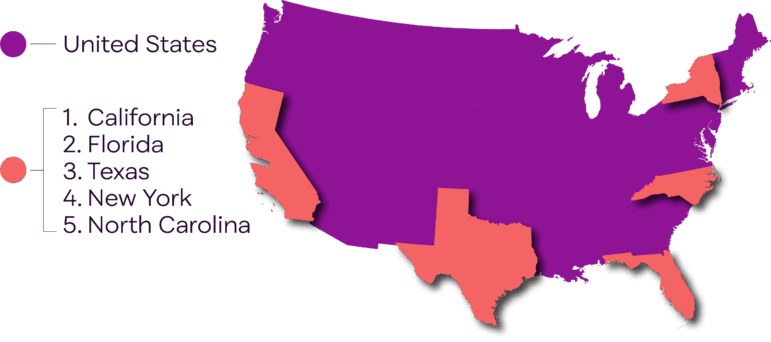
Clinical trials can be found in every state. But the top locations, as shown in the map, are states with more people. This limits diversity. And that means researchers aren’t learning enough about how a potential treatment might affect some of us. To address this problem, trials need to include more people from less populated states. The best way to do that is to have more sites in rural areas.
What else do I need to know?

Clinical trials and race
Find out how the history of clinical trials impacts medical mistrust today.

Clinical trials, sex, and gender
Understand some of the history of clinical trials related to gender and biological sex, and why representation is key.
Find a clinical trial
PAN’s TrialFinder site makes it easy to search for clinical trials based on your condition and location.
Call us for help
Our ComPANion Access Navigators can answer your questions and help you use our trial finder.
1-855-329-5969
Stay connected
We do more than just clinical trial education. Sign up to receive news and updates from the PAN Foundation.
