Clinical trials, sex, and gender
Clinical trials are more gender inclusive today, but we are still closing the gap.
Over the past three decades, clinical trials have become more inclusive of biological sex and gender. Trials still need more representation of females and all gender identities.
History of gender bias and exclusion in clinical trials
Women only make up about 41% of clinical trial participants, despite being more than half of the world’s population.
Did you know that it was not until 1993 that the inclusion of females in clinical trials was required by law? Females were deemed “too complex” for trials. This was based on biological makeup, including hormonal changes and the potential risks in childbearing. It’s hard to believe, but small males were used to determine if many of the treatments we use today would be safe for females.
The LGBTQ+ community has also been left out. Some trials exclude medications used for gender-affirming care. Many trial teams are not trained to ask key questions, and facilities at trial sites are often outdated. These and other barriers keep too many of us from joining trials.
Timeline of gender and clinical trials
1860
Pre-American Civil War
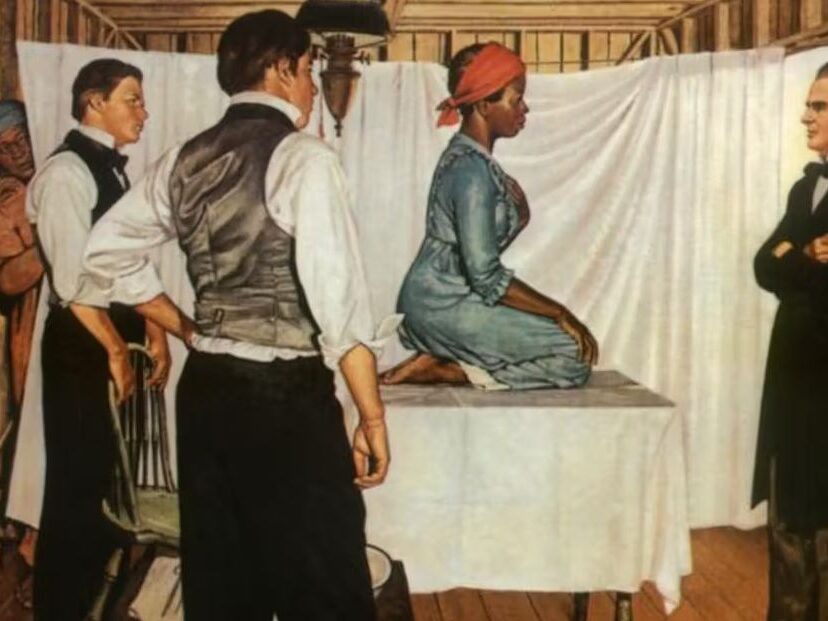
1919
Early 20th century

1930
Experimental sex reassignment

1950s
Progesterone, birth control studies

1962
Thalidomide tragedy

1977
Exclusion of females in clinical trials
1993
NIH Revitalization Act

2009
Caring for transgender patients

2019
Transgender redefined

Clinical trials today

Females in clinical trials
Females make up 51% of the U.S. population. But they make up only 41% of clinical trial participants. Over the past three decades, the gap has narrowed. But it’s still too large.
The only way to know how a potential treatment or vaccine might work in females is to test it in females. That’s why we need to increase female participation in trials. Ways we might reach this goal include:
- Travel support for trial visits
- Flexible trial visit schedules
- More women on trial teams
- Support for childcare needs during trial visits
Building trust in clinical trials
To build trust, trial sponsors and teams can engage with a wide variety of communities and understand their concerns. They can address social health factors and offer educational resources that help all of us understand trials. We’re headed in the right direction, but there’s a long way to go.

Clinical trials for health conditions that affect females more than males
The participation gap in clinical trials has caused a gap in healthcare. In many cases, we don’t know as much about how potential treatments and vaccines will work in females. This can pose health risks. And the risks can be magnified for health conditions that affect females at a higher rate.
Illness can affect females in different ways. For example, females have a higher rate of death after a heart attack. Other health conditions that affect females more frequently include:
- Breast cancer
- Depression
- Lung cancer
- Menopause
- Urinary tract illnesses
More focus is needed on gender identity
We all deserve the right to live our healthiest lives. And it starts with inclusive clinical trials. Though the numbers vary by study, about 9 percent of adults in the US identify as LGBTQ+, and millions identify as transgender and/or nonbinary.
Bias faced by people in these groups can lead to medical mistrust. They may think trials are not for them. But that does not mean they should be left out. According to one report, less than 1% of clinical trials in the United States include people who identify as nonbinary or transgender—but this group represents about 1% of the population.
Sponsors have worked to improve trial access for all. But efforts have focused largely on race and ethnicity. Trial sponsors should take additional steps to include the LGBTQ+ community. For example, sponsors can:
- Consider negative experiences the LGBTQ+ community has faced and work on building trust
- Ask participants about their gender identity
- Find more trial sites that have inclusive facilities
- Include gender-neutral language and images in trial-related materials
- Train trial teams to be sensitive and inclusive (including asking about and using a person’s pronouns)
- Engage advocacy groups for input on trial design
- Recognize and remove barriers to study participation

Facts and fiction: a look at misconceptions
When it comes to clinical trials, it’s important to separate fact from fiction. This can help you make a more informed choice about participating. Let’s start by clearing up two misconceptions about clinical trial gender bias today.
Bias and Exclusion
FICTION: LGBTQ+ people are not allowed to join clinical trials.
FACT: Most trials don’t exclude people based on gender identity or sexual orientation. All trials have criteria for who can be in them. But an Institutional Review Board (IRB) reviews the criteria. The IRB makes sure you won’t be kept out of a trial unless there is a reason based on science.
Trial Participants
FICTION: Most clinical trials are for male participants only.
FACT: In the past, clinical trial participants were almost all white males. But that changed dramatically in the early 1990s. The NIH Revitalization Act of 1993 requires that females be included in trials funded by the U.S. government. Other trial sponsors adopted this new approach. Trial teams now look for people who match all patients who may benefit from a potential treatment or vaccine in the future. For most trials, this means they are looking for females and males in equal numbers. A recent study found that 41 percent of trial participants are female.
Find a clinical trial
PAN’s TrialFinder site makes it easy to search for clinical trials based on your condition and location.
Call us for help
Our ComPANion Access Navigators can answer your questions and help you use our trial finder.
1-855-329-5969
Stay connected
We do more than just clinical trial education. Sign up to receive news and updates from the PAN Foundation.
